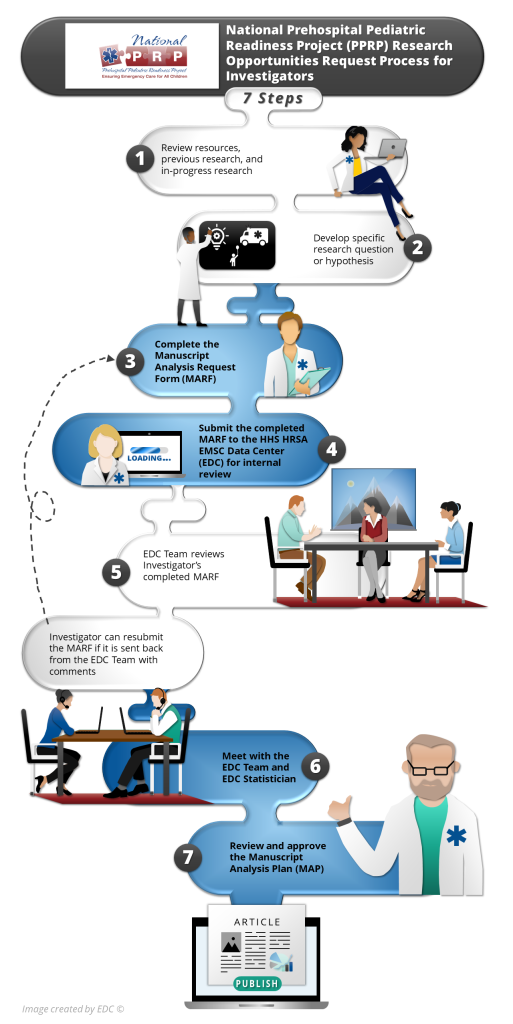
PPRP Research Opportunities Request Process
This image and the summary below outline the steps and forms to submit a research question/hypothesis to the HHS HRSA EMSC Data Center (EDC).
After identifying a research question, the Investigator reviews the published papers associated with the Prehospital Pediatric Readiness Project, the 2024 summary statistics, the data dictionary, list of in-progress research projects, and a PDF version of the 2024 Prehospital Pediatric Readiness Assessment. These resources and documents will not be available until 2025, after the 2024 assessment closes.
The reason for the review is for the Investigator to assess if their research question can be answered with the available variables and to ensure that the question has not already been or is currently being investigated.
The next step involves the Investigator developing a specific research question or hypothesis.
Using their research question or hypothesis, the Investigator completes the Manuscript Analysis Request Form (MARF). This form helps the Investigator consider various aspects of the research.
The Investigator submits the completed MARF to the HHS HRSA EMSC Data Center (EDC) for internal review. We will not be accepting MARFs until after the 2024 PPRP Assessment closes. At that time, Investigators will be able to email the completed MARF to the EDC Team.
The EDC Team reviews the completed MARF. The review process takes 2+ weeks. There are three review outcomes
- Accepted
- Comments and Resubmit
- Not Accepted
If the MARF is accepted, the Investigator continues with the process. At this point, an EDC Statistician will be assigned to the project at no cost to the Investigator.
The Investigator can resubmit the MARF if it is sent back from the EDC Team with comments and the Investigator wishes to revisit and modify the research question. There may be times when the EDC Team feels that the research question/hypothesis is not suitable or needed at that time and the request will not be accepted. In these cases, the Investigator will not be asked to resubmit a MARF.
Once the EDC Team accepts the Investigator’s MARF, a meeting or telephone call is scheduled between the Investigator and the EDC Team and EDC Statistician. The EDC Statistician completes a Manuscript Analysis Plan (MAP) based on the discussions. The MAP is a more detailed version of the MARF with the variables and analyses specified.
The Investigator reviews and approves the MAP. In most cases the EDC Statistician conducts the analyses by following the MAP (see EDC Statistician Analyses Process below) and shares data tables with the Investigator.
In limited cases, an encrypted password-protected dataset may be sent to the Investigator (see Investigator Analyses Process below), after a Data Use Agreement (DUA) is approved by the Investigator’s institution and the University of Utah – the DUA will be initiated by the EDC Team. The Investigator will then conduct the analysis.
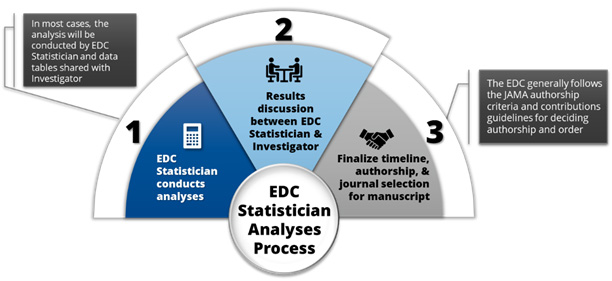
EDC Statistician Analyses Process
EDC Statistician conducts analyses per approved MAP
Results discussion between EDC Statistician and Investigator
Finalize timeline, authorship, and journal selection for manuscript. The EDC follows the JAMA authorship criteria and contributions guidelines for deciding authorship and order
Investigator Analyses Process
- Final approval from the Investigator’s institution’s IRB received by EDC Team
- Investigator and University of Utah institutions complete a Data Use Agreement (DUA)* – the DUA will be initiated by the EDC Team. This step can take 6 weeks to 2 months
- When approved, the Investigator receives an encrypted password-protected dataset
- Results discussion between EDC Statistician and Investigator
- Finalize timeline, authorship, and journal selection for manuscript. The EDC follows the JAMA authorship criteria and contributions guidelines for deciding authorship and order
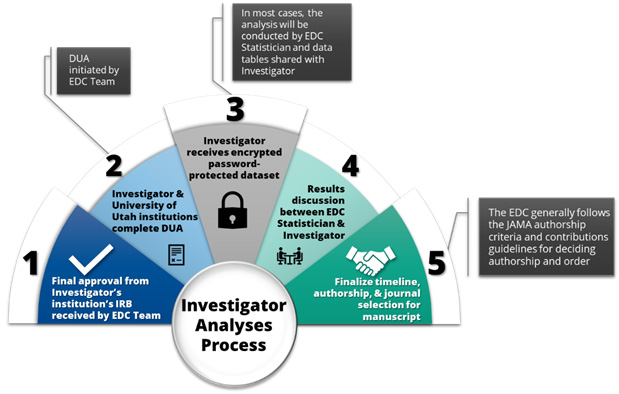
*In most cases, the analysis will be conducted by a statistician at the EDC and the data tables will be shared with the Investigator. However, there may be times when the investigator conducts the analysis. In this case a DUA is needed before an encrypted password-protected dataset is sent to the investigator. The dataset provided through a DUA requires approval by recipient investigators’ IRB or demonstration of exemption from the need for IRB approval by institutional policy. Dataset creation and distribution for the DUA will be performed by the EDC. The EDC will provide the dataset and data dictionary. The recipient investigator will be responsible for maintaining confidentiality of the dataset and the analysis.